After the positive evaluation of our Quality Management System according to the IVDR Regulation 2017/746 in 2022, and the obtaining of our first CE markings according to the same regulation in March 2023, DIAGAST takes another key step in its IVDR project by obtaining the first IVDR certification for class D products.
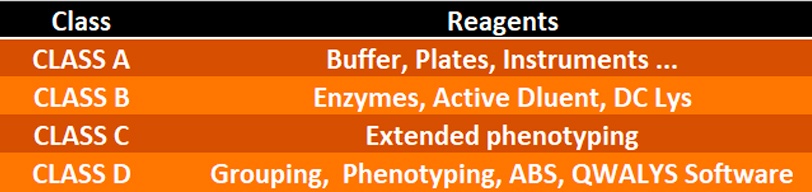
The class D products concerned are the so-called ̈Anti sera PK ̈ reagents, developed to carry out the:
• 2 grouping determinations
• 2 phenotyping determinations
on the Beckman Coulter ̈ PK7300 and PK7400 automatons.
The new IVDR Regulation ensures that ¨in vitro¨ diagnostic devices manufactured for sale in the EU are assessed against strict quality, safety and performance requirements. DIAGAST, like other manufacturers, is required to provide, inter alia, substantial evidence of scientific validity, as well as data demonstrating the analytical and clinical performance of the devices.
This is only one step! The IVDR project continues to certify all our products to ensure a high level of performance and safety.
Thank you to the teams involved on a daily basis in this major project for our company. It is everyone’s investment and strong collaboration that make and will continue to make our success.