External Quality Controls
According to the French law L.6221-9 and the ISO 15189 standard, External Quality Assessment (EQA) programs are mandatory for French laboratories. They are an objective proof of the quality of the analyses performed in your laboratory.
That is the reason why DIAGAST has teamed up with the U.C.I.L. (Interlaboratory Control Unit) of the French Blood Service (EFS), to offer you a large range of unknown results samples, whatever your technique and equipment..
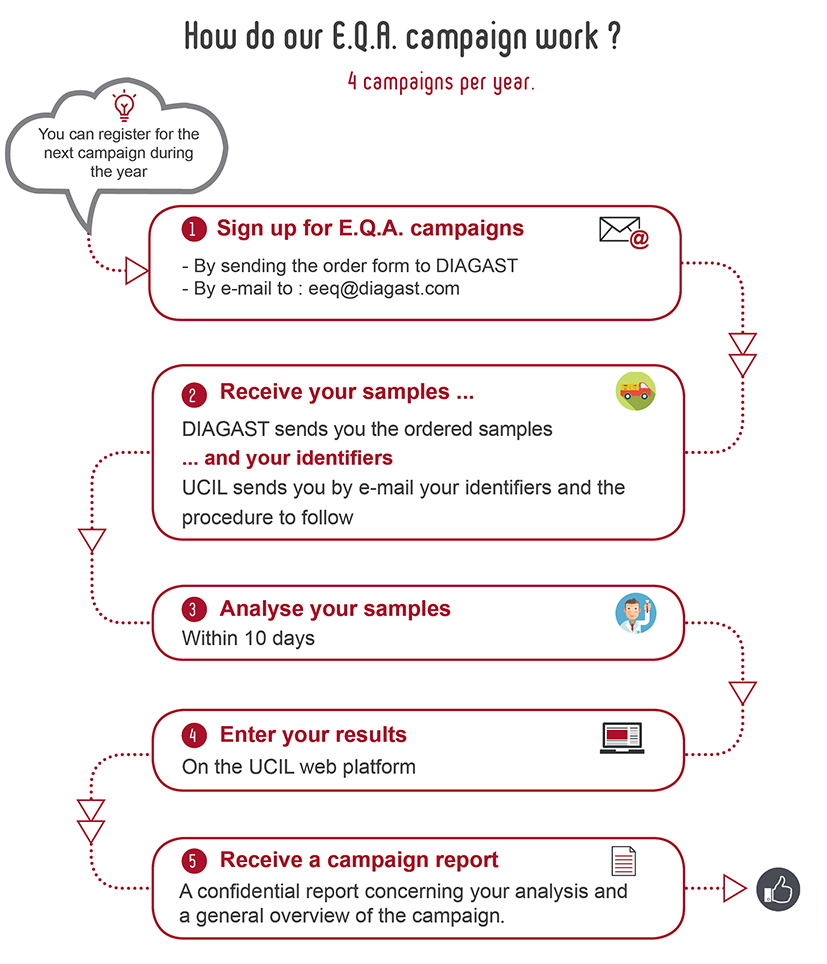
Every year, the U.C.I.L. prepares 4 campaigns of External Quality Assessment.
DIAGAST guarantees complete transparency of the external control campaigns. DIAGAST has no knowledge of the test results for each campaign, nor of the confidential reports sent to each participant.
| EEQ 1 – For grouping, phenotyping & AntiBody screening | 59511 | |
| EEQ 2 – For Direct Antiglobulin Test | 59512 | |
| EEQ 3 – For extended phenotyping | 59513 | |
| EEQ 4 – For crossmatching | 59514 | |
| EEQ 5 – For elution test | 59515 | |
| EEQ 6 – For Antibody titration | 59516 |